Proud provider of customized, multi-channel medical education to ~25% of community oncologists in the United States
IDEOlogy Health
For Our Strategic Partnerships
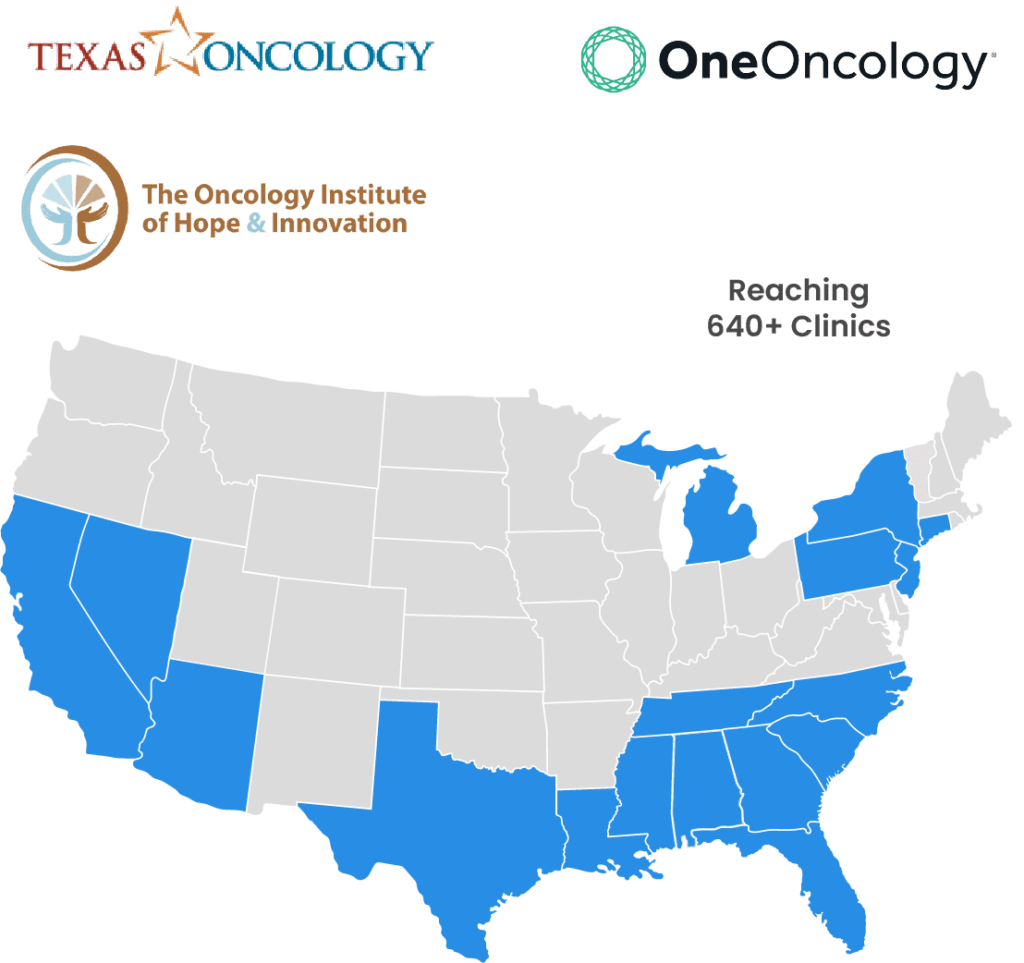
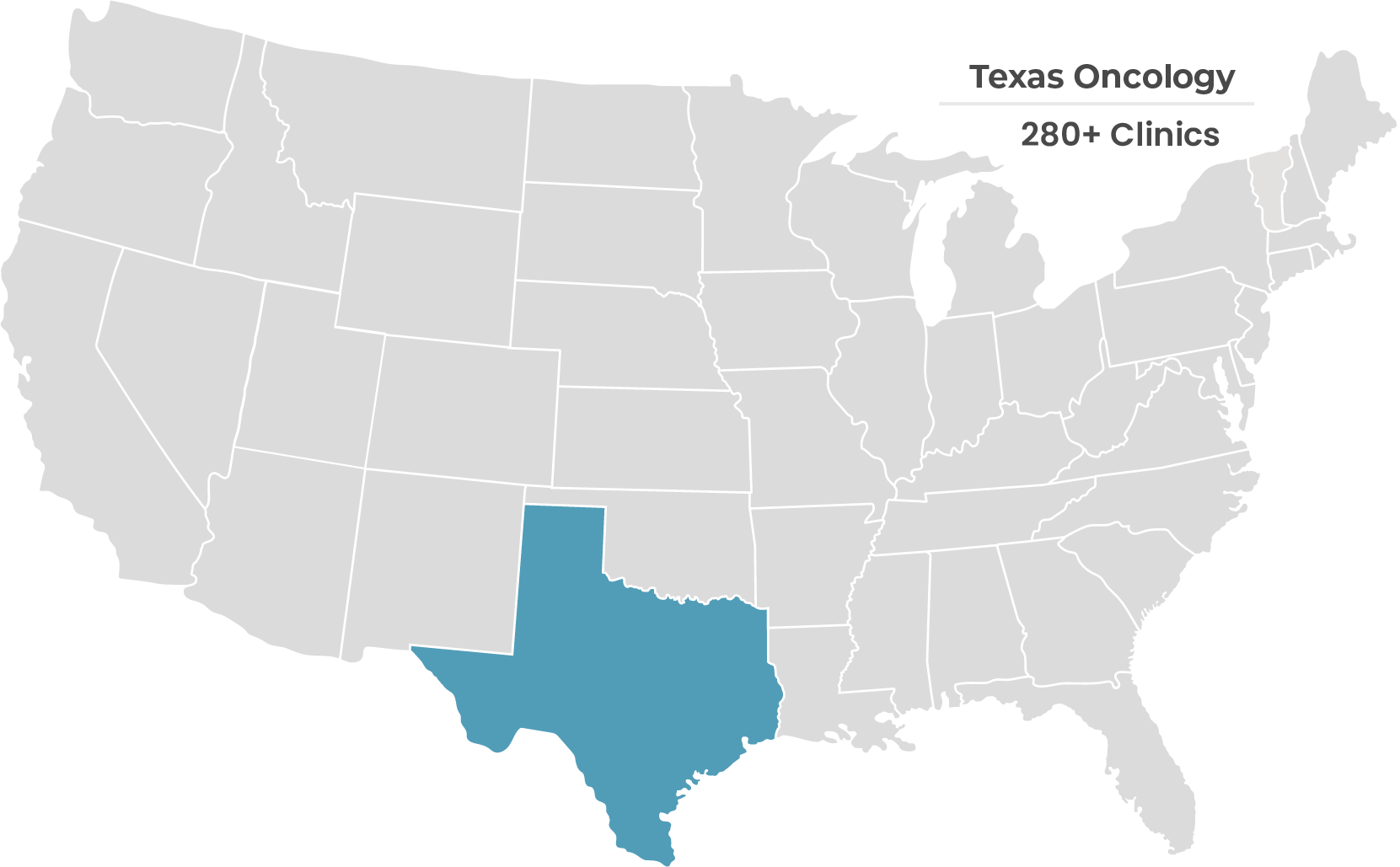

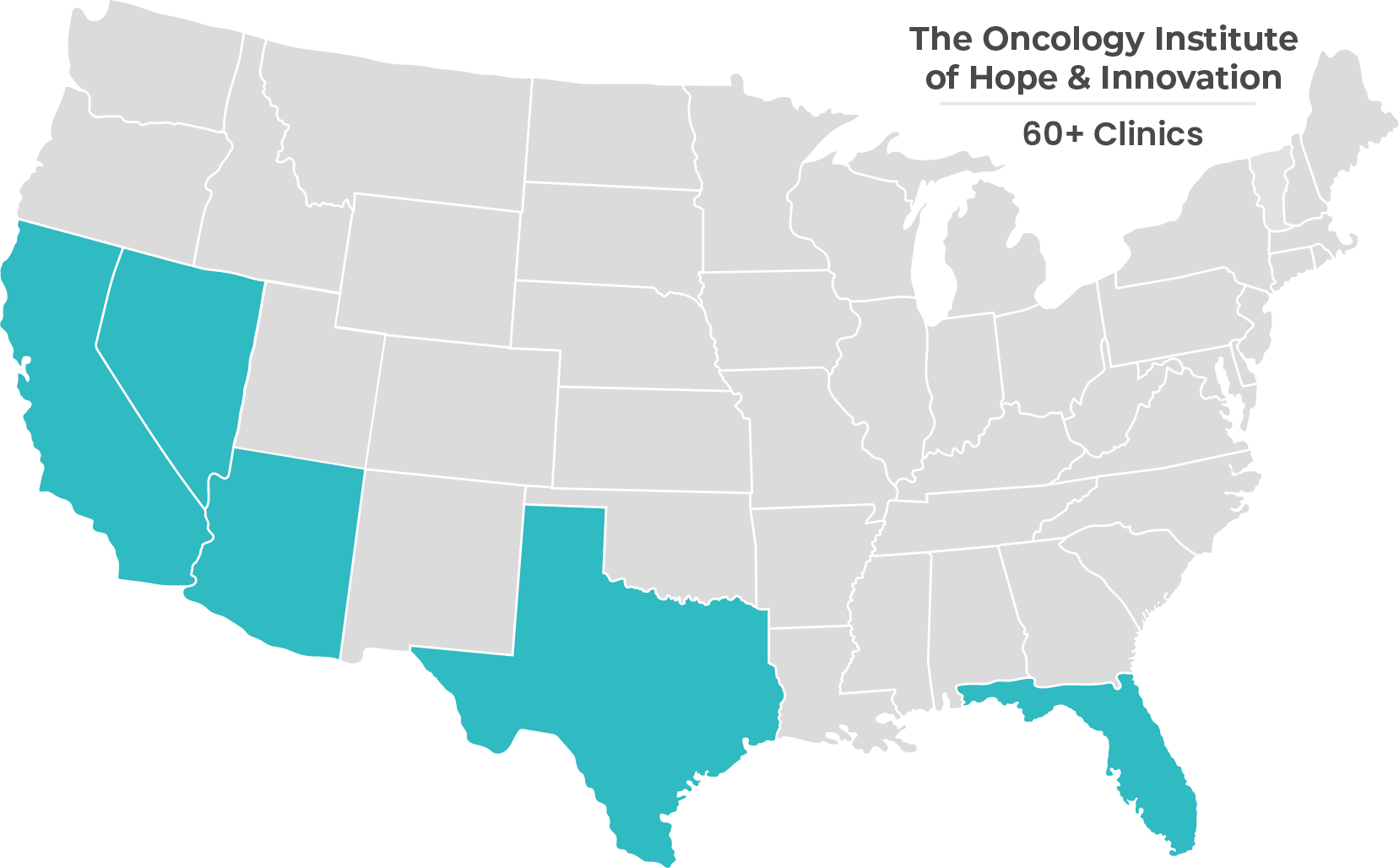

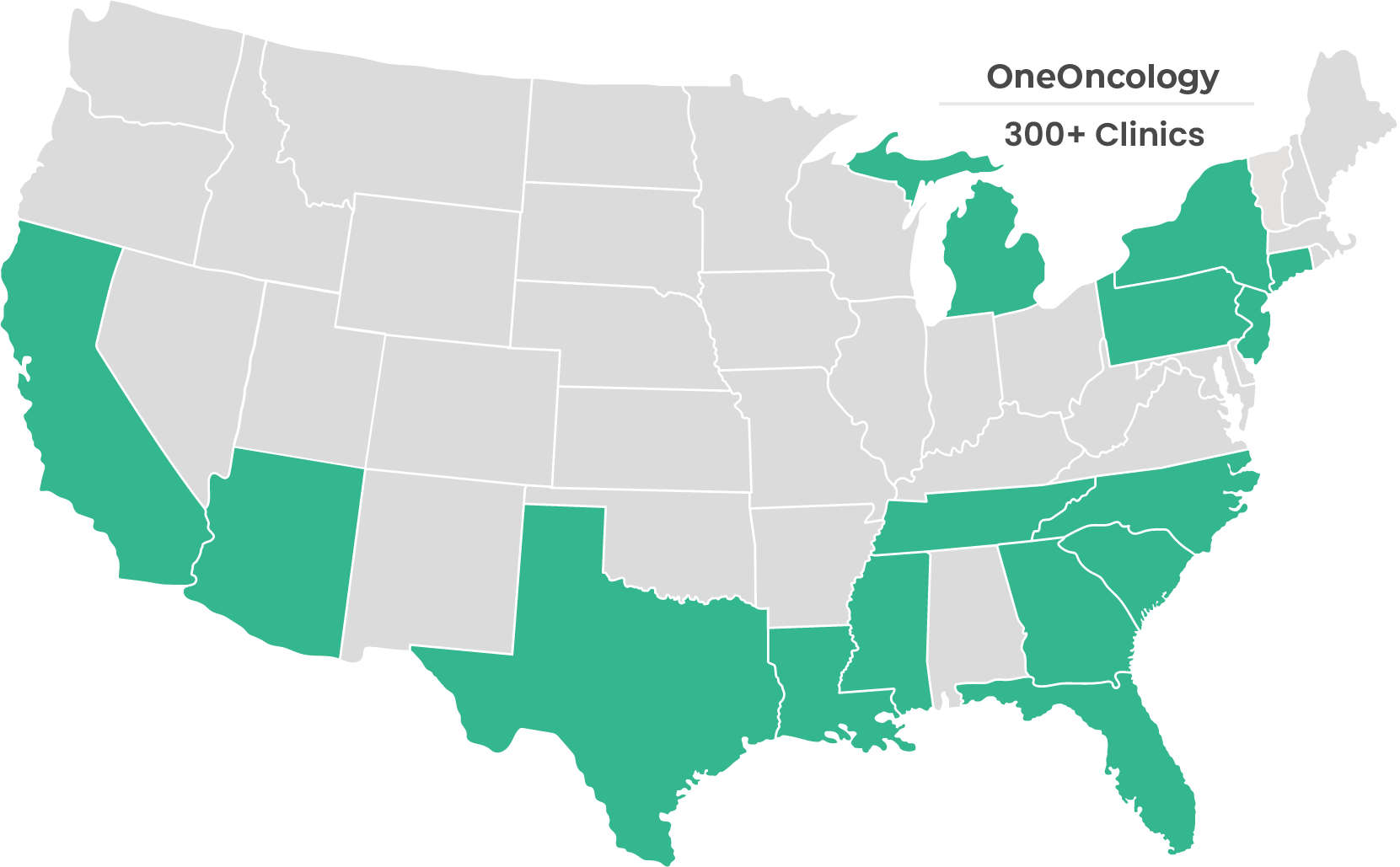

Hear From Our Strategic Partners






IDEOlogy Health
For Community Oncologists


IDEOlogy Health hosts unbiased roundtable discussions that connect leading experts with community oncologists to stay up-to-date on the latest clinical data.


National, virtual programs with world-renowned oncology thought leaders discussing the latest post-congress data.
IDEOlogy Health
For On-Demand Updates


Convenient, non-promotional, short video clips from distinguished thought leaders on the latest clinical data sent directly to you via text message.
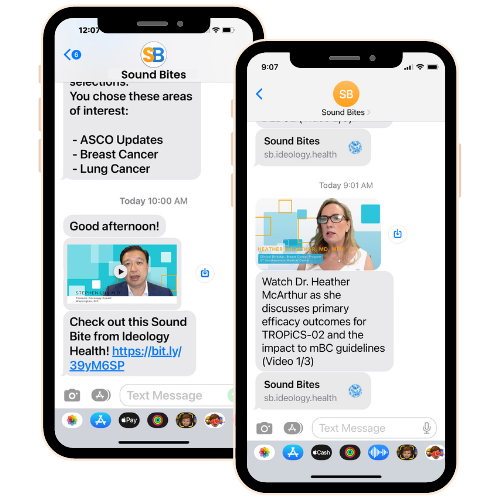

IDEOlogy Health
For Continuing Education


The 2024 Texas Lung Cancer Conference in Austin, Texas, on April 18-20, 2024 will bring 50+ of the top minds in lung cancer together with community oncologists from across the country to share, inspire, and move the science forward in thoracic oncology.


The World Conference On Genitourinary Cancers in New York City, NY, on August 1-3, 2024 will be chaired by five world-renowned thought leaders in GU cancers.
Contact Us
Contact us to join our network, learn more about our educational offerings, or inquire about joining our faculty. We are your source for the latest in disease-state solutions.
info@ideologyhealth.com
